About Classical Theories of Venous Return
About Classical Theories of Venous Return
Our purpose here is to review the current state of knowledge concerning the physiology of venous return. It is accepted that the heart ensures the return of blood from the periphery to the centre. However, as we will try to show:
The heart does not intervene in the venous circulation
In this regard, we will quote this excerpt from the website of the French Federation of Cardiology:
“The blood pressure due to cardiac contractions does not intervene in this second part of the journey and venous return is achieved only by the veins themselves.”
The forces at work
Here we will give a brief review of circulatory physiology. This includes a pump, the heart, and vessels, arteries and veins. One factor to be taken into consideration is gravity, especially in the standing position. For all the vessels below the heart, gravity ‘helps’ the blood flow to the periphery, while it is a brake on the return of blood to the centre. This is particularly noticeable for the lower limbs. Let’s look at the physiological elements involved.
The pressures
At the level of the great circulation, the heart assumes the movement of blood in the arteries, arterioles, capillaries and venules. To do this, the left ventricle contracts, creating a pressure of 120 mm Hg. At the exit of the capillary, the remaining energy is only 1.5 mm Hg.
We deduce from this that at the exit of the venules, an additional force is required to ensure the return of blood to the right atrium.
The volumes
Let’s look at the differences between veins and arteries in terms of their number, size and volume. Veins are twice as many and twice as big as arteries.
Regarding the difference in volume: The volume of a cylinder is equal to π r2 x h
The volume of blood contained in the veins will therefore be 8 times greater than that contained in the arteries.
In the standing position, the arterial circulation benefits from the help of gravity. At the height of the foot, the weight of the blood column exerts a pressure of 90 mm Hg for every 120 mm Hg produced by the heart, i.e. 210 mm Hg. Unlike the arterial system, gravity represents an obstacle to the return of blood to the veins.
From the 1.5 mm Hg remaining at the capillary exit, the 90 mm Hg corresponding to the weight of the blood column must be subtracted this time, resulting in a negative balance of 88.5 mm Hg. In other words, in order for the blood to return to the heart, 88.5 mm HG plus the force necessary for the blood to flow back up into the veins is missing.
How can this pressure deficit be justified? How can we explain the return of blood from the feet to the heart when we are standing?
Let’s look at the commonly accepted physiological responses. Four forces are described that favour venous return:
Translated with www.DeepL.com/Translator (free version)
- The tergo screw
- The front screw
- The side screw
- The muscle pump
1. The tergo screw (Richard Lower, 1670)
It is defined as follows: the weight of the arterial column continues to push the blood after passing through the capillary, which would explain the blood’s ascent into the veins. The circulation would resemble a U, with a descending branch, favoured by gravity, and an ascending branch, where the circulation would take place thanks to the thrust coming from the descending branch.
Translated with www.DeepL.com/Translator (free version)
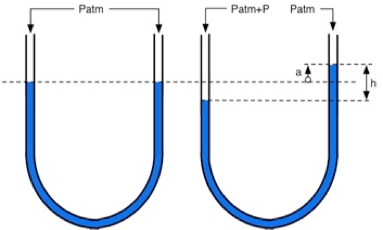
Fig 1: The arterial-vein system forms a kind of U-tube, in which the pressure due to the weight of the liquid column is the same at each of the points of either branch located on the same horizontal line. »
However, a high venous pressure value at the extremities of the lower limbs remains compatible with normal venous return through the simple mechanism of the tergo screw. Indeed, in the tibial malleoli, the venous blood column exerts a pressure of 90 cm of H2O, solely due to gravity, if this is the difference in level between the heart and the malleolus. But the same effect of gravity is exerted in the arteries and arterioles located on the same horizontal plane (1). Consequently, the pressure which ensures the flow of blood in the capillaries is always equal to the arteriolar pressure minus the pressure in the venules, but both being, due to gravity, increased by 90 cm of H2O. The screw a tergo (pressure in the venules remaining from the cardiac pulse) is therefore also increased by 90 cm of H2O.
Excerpt from the physiology book Hermann Ciers
This hypothesis can be contested, since the arteriovenous system does not constitute a homogeneous U, since the capillaries exert significant resistance at the end of the descending branch of the U.
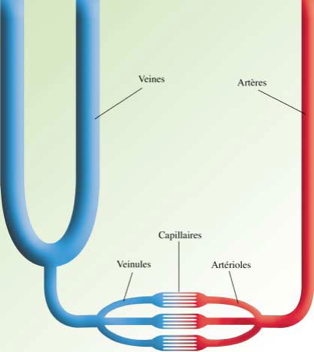
Let’s see FIG 2 the anatomical reality of this U.
In the limbs, the veins are twice as numerous and twice as large as the arteries.
The ascending branch of the U (the venous column) therefore has a volume eight times greater than the descending branch (the arterial column).
Poiseuille’s law allows us to calculate the forces present. Taking into account the difference in volume and size between veins and arteries, for arterial blood to push venous blood to the right atrium, it would require a force 32 times greater than that exerted by the left ventricle.
If the screw a tergo were a clinical reality, a simple injury to the veins of the foot would empty us of our blood in a few seconds (there would also be propulsive pressure in the saphenous veins, which is not the case). The tergo screw therefore takes into account neither the specific anatomy of the vessels of the limbs, nor the resistance exerted by the capillaries, nor the load exerted by gravity .
It can be said: The tergo screw cannot explain the blood flow up to the right atrium.
2. The front screw
These are forces from the heart and the diaphragm that draw blood into the central veins.
For the heart
The aspiration exerted by the contraction of the auricles was described by Antonio Valsalva in 1710.
During diastole, a negative pressure of – 2.2 mm Hg is measured in the right atrium creating a suction effect of the blood contained in the vena cava. This force is nowadays considered to fill the atrium but is inoperative beyond this point.
For the diaphragm
There would be aspiration of blood into the inferior vena cava on thoracic diaphragmatic inspiration. The mediastinal pressure would then become negative due to pulmonary amplification. The inferior vena cava would then be rhythmically dilated, producing aspiration of the blood contained in the inferior subdiaphragmatic vena cava.
What about this proposal?
Anatomical reminder
The length of the lower intramediastinal vena cava (ICV) is approximately 2 cm. Its diameter is 2.2cm The volume of this cylinder is therefore (r2 x L x π) or 1.12 x 2 x π = 7.6 cm3
The theory of the intramediastinal face screw therefore applies to a volume of about 7.6 cm3.
It is difficult to calculate the exact amount of blood flow to the heart due to this force. It would be equivalent to the difference in volume of the inferior vena cava in its supra-diaphragmatic portion during inspiration and expiration.
The reduction in caliber of the ICV during the exhalation and inhalation phases is about 50% of its diameter. The volume of this diameter reduced by half will then be 0.6 x 2 x π = 3.7 cm3 .
The volume differential would then be 7.6 – 3.7 = 3.9 cm3 every 4 seconds. That is nearly 1 cm3 per second.
The heart, at rest, ejects 600 cm3 of blood per minute, or 10 cm3 per second. More than 55% of the blood volume will return through the inferior vena cava. Let 600 x 0.55 = 330 cc. Let: 330 / 60 = 5.5 cm3
The possible assistance provided by the face screw would represent less than 20% of the flow of the VCI at rest .
The difficulty of such an experiment is that during inspiration the lowering of the diaphragm compresses the contents of the abdomen as a whole. At the moment of inspiration, if there is a mediastinal depression which (weakly) sucks up the blood under the diaphragm, there is also at the same time a not inconsiderable inflow of blood from the space under the diaphragm.
In fact, the Lower Sub-abdominal Vena Cava is compressed during diaphragmatic inspiration and flattens. Diaphragmatic inspiration therefore flattens the ICV located mainly under the diaphragm and has almost no chance of dilating the supra-diaphragmatic portion of this ICV which is almost non-existent above the diaphragm.
Echo-doppler experiments on the normal compliance of the ICV give contradictory results.
The compliance of the inferior vena cava is studied by examining its variation in caliber during the respiratory cycle. Normally, the diameter of the ICV is reduced by at least 50% during inspiration. The clinic here invalidates the theory of the mediastinal screw front.
The above figures take into account a compliance differential of the VCI by compression of the VCI. Of course, a compliance differential of the VCI by diaphragmatic depression would have a much smaller effect. Less than aspiration of the right atrium, which is classically considered ineffective for venous return.
We also know that :
- Open chest procedures don’t prevent blood from flowing back to the heart.
- The best apneists remain up to 24 minutes without breathing and the circulation does not stop for all that.
We can therefore conclude that the front screw, like the tergo screw, cannot explain the rise of venous blood.
3. The side screw
This force concerns the lateral pressure exerted on the veins. There are two such effects: compression due to muscle masses, and compression due to the arteries.
Lateral muscular compressions
The contraction of the muscles thickens the muscle mass, expelling blood into the veins that lie outside. The diameter of the vein is reduced by this lateral pressure, propelling the blood upwards, the valves preventing a retrograde flow (fig 3).
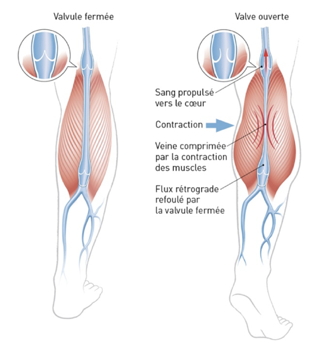
Fig 3 The side screw
This theory concerning twins (gastrocnemius) is far from being an anatomical constant, it is even a regional peculiarity.
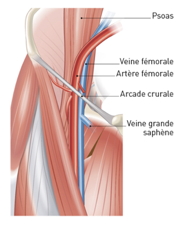
Fig 4 : Indeed, at the level of the femoral arch, when contracting, the psoas would press on the femoral vein. This effect is prevented since the vein is separated from the muscle by the femoral artery. This time the femoral vein is protected from the psoas contraction by its homologous artery.
Lateral compression by the vessels
At the time of cardiac systole, the arteries dilate. This expansion compresses the satellite veins, promoting venous return. Recent studies (echo-doppler) have shown that the venous flow is linear, unlike the flow of arterial blood, which is rhythmed according to the cardiac contractions. We do not therefore find a pulsed flow as would be suggested by a lateral screw coming from the arteries.
The sole of Bourceret
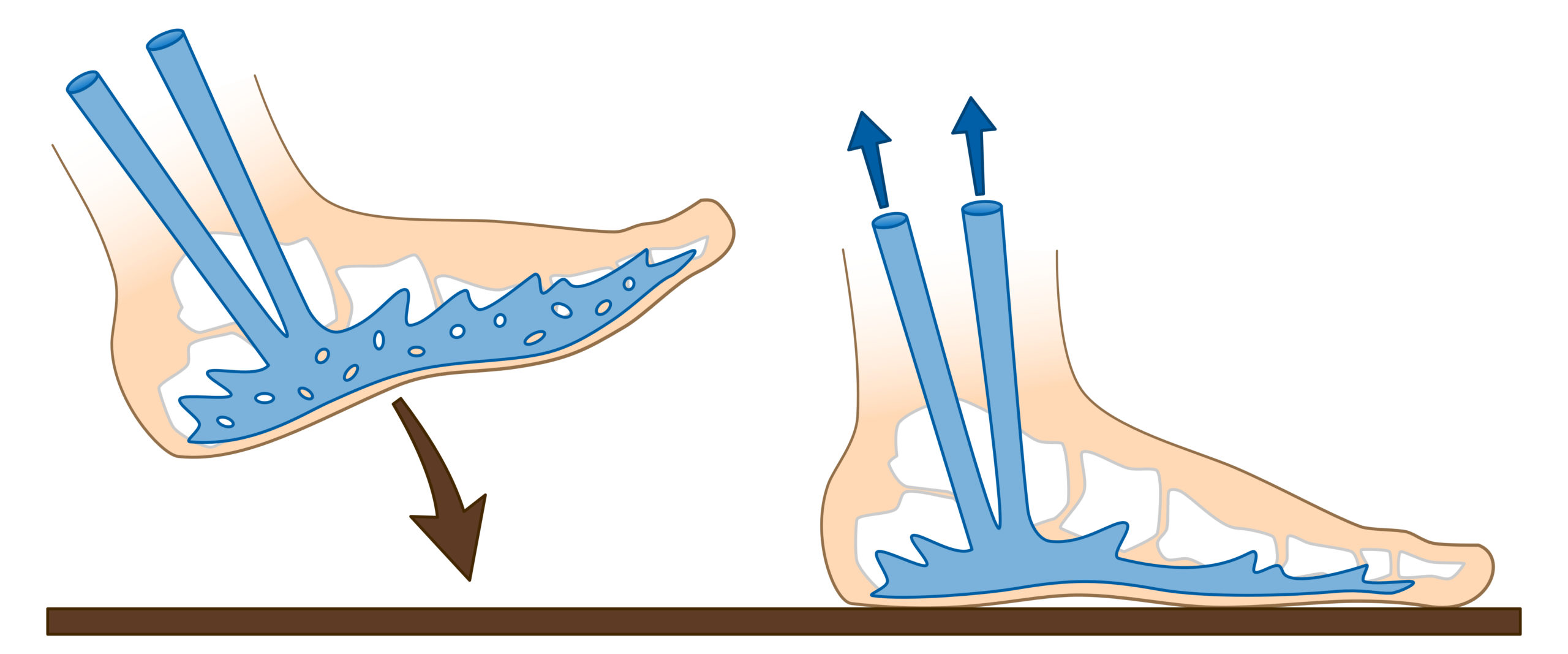
The support of the foot on the ground when walking flattens the sole of Bourceret, driving venous blood to the calf veins. This mechanism is considered effective.
4. The muscle pump
Knight of Richerand, in 1817
It’s the most recent theory about the motors of venous return. The increase in pressure of the intramuscular venules is then multiplied by 5 during muscle contraction. The muscles, by contracting, expel blood from the veins located in their mass.
This is demonstrated during a Doppler examination.
In total, among all the mechanisms proposed to explain venous return from the periphery, two main ones can be retained: the muscle pump (isotonic contractions) and the pumping of the Bourceret sole during walking).
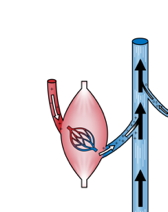
The contraction of the muscle expels the blood it contains into the veins, even at rest.
The venous return in seedlings who are immobile is explained by static contractions in their calves. Such contractions cannot ensure a pumping effect. Indeed, a pump is defined as a mechanism with an emptying time and a filling time, which does not occur with static contractions.
These mechanisms are undeniable but do not fully explain venous return from the lower limbs, as can be seen in certain extreme situations, such as the Queen’s Guards maintaining an immobile standing position, or paraplegics maintained in an upright position.
We therefore need to find a return mechanism, since, as we saw above, the power of cardiac pushing or suction is almost negligible in the lower limb venules.
Current physiological theories therefore avoid two important phenomena concerning venous return:
- What is this motor powerful enough to bring venous blood back to the right heart?
- What is the mechanism that makes the venous flow linear? Current theories propose solutions with rhythmic flow rates.
It is therefore up to us to find a new return mechanism with a linear flow, since, as we have seen above, the power of cardiac pushing or suction is almost negligible in the lower limb venules and the proposed solutions are rhythmic.
